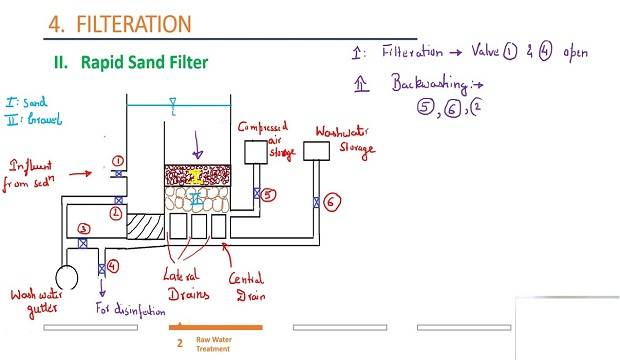
Rapid sand filter
The loss of head in the filter operation is caused by the frictional resistance offered by the filtering medium and the underdrains to the water flow.
The value of the head loss is equal to the vertical distance between the water level on the filter bed and the elevation of the hydraulic grade line at the filter outlet.
The loss of head immediately after washing should not be more than 10-15 cms. When sand bed gets choked up with impurities the rate of discharge falls down and it is necessary to wash it to regain its original clean conditions and the stipulated rate of discharge.
In the filters provided with loss of head indicators this choked-up condition is shown by the increase in the loss of head so that when it reaches a certain figure generally about 1.8m the filter requires washing.
When the loss in the top layers of the sand becomes greater than the head of water above the sand bed (due to choking) and the water column below such layers acts as a draft tube and a partial vacuum is created.
This condition is known as negative head. Thus the negative head at any point in a filter is equal to the intensity of vacuum at the point and is usually a maximum at the point where the water enters the under drainage system.
Explain in detail the working of rapid sand filter
The pretreated water from the coagulation sedimentation tanks through the inlet Billeni valve is admitted into the filter units. It is distributed by the troughs which remain zi sont submerged while working over the entire bed area.
The water then percolates me through the sand and gravel layers and thereby the fine suspended and colloidal impurities present in it are arrested by the sand layer. After this, it enters the laterals through the strainers or holes and then leaves the filter unit via the manifold.
In the beginning, the loss of the head of the filter is about 15-22cm. As the filtering media goes on getting clogged with arrested impurities, the resistance to the passage of water and hence the loss of head increases. Usually, a maximum loss of head 2- 2.5m is permissible.
If it increases further till it may pack the sand so tightly as to cause difficulties in back washing or may cause the water to break through sand without filtration. The rapid sand filter unit is then washed, the clogging is removed and normal working resumed
Explain in detail the construction and working of slow sand filter.
The slow sand filter consists of open tanks 2000to 4000sq. m in plan and 3 to 4 deep, containing 600-900mm thick bed of sand supported on 300mm thick gravel layer.
The effective size of sand is 0.35mm with a uniformity coefficient of about do amol 1.75. The top of 150 mm layer of the filtering media is of a finer variety 0.25mm, the gravel size varies from 4.7 to 12mm and is placed in layers with the smallest size orto particles at the top and the largest on the bottom.
Treatment of water ! Slow sand filter ! Filtration of water
The sand and gravels are laid over a system of open jointed underdrains placed 3 to 6 m apart on the bottom floor sloping towards a main covered drain constructed along the center or side of the filter tank.
Settled water from the plain sedimentation tank is distributed uniformly over the filter bed. It percolates through the sand bed and the gravel and gets purified during the process of filtration.
The under-drainage system collects the filtered water and passes it on to the clear water reservoir. As the filter media gets clogged the resistance to the passage of water and the head loss increases.
The water in the filter tank is first drained, and the thick layer of impurities collected over the surface is then scraped and washed clean with water jets from hoses. The filter is then put back into normal operation.
Name various disinfecting methods and explain any one of them in detail. Various disinfecting methods are as follows.
1. Chlorination are con
2. Ozonization
3. Ultraviolet ray method
4. Excess lime process
5. Application of silver
6. Iodine or bromine
7 Potassium permanganate
Ozonization-The effectiveness of ozone in the disinfection of water lies in its high oxidizing power. Ozone is an unstable isotope of oxygen-containing three atoms of oxygen which while changing to the stable molecular form O, releases nascent oxygen.
The nascent oxygen reduces organic matter present in the water without the production of objectionable tastes and odors as with chlorine. The ozone dose is 2-3mg/l to give a trace of 0.1mg/l residual after 10 minutes of contact.
Ozonization is regarded as a natural means of disinfecting water and is particularly useful in disinfecting water containing bacterial spores. It is however costly to manufacture, has very little residuals present, and is not quite suitable for highly turbid waters
What do you understand by chlorination? Explain its action in killing of bacteria.
The process of applying small quantities of chlorine or chlorine compounds to water is called chlorination. The chlorine dose applied is generally 1ppm so as to produce residual chlorine of quantity varying from a trace to about 0.05-0.2 ppm.
The chlorine demand is defined as the difference between the amount of chlorine added and the amount of chlorine remaining at the end of a contract period of 15-30 minutes.
Action of chlorine:
Chlorine reacts with water to produce hypochlorous acid (HOCI) and hypochlorite ion (OCI), which are together known as free available chlorine. The chemical action may be represented as
CI, +H, O = HOCI + HCL
HOCI= H + OCI
If ammonia is also present in water, the other compounds formed are monochloramine (NH,CI) and Dichloramine (NHCI) which are together known as combined available chlorine.
These resulting chlorine compounds either in the form of free or combined available chlorine interfere with certain enzymes in the bacterial cell wall forming a toxic chloro compound thus destroying the bacteria completely.
The effect of chlorine as a disinfectant is principally dependent upon the period of contact and the concentration of chlorine in the water.
Explain chlorine ammonia treatment for disinfecting drinking water. What are its advantages of it.
Use of chloramines or use of chlorine with ammonia Chloramines are the disinfectant compound which are formed by the reaction between ammonia and chlorine.
These compounds are quite stable and remain in the water as residuals for a sufficient time.
Hence they can produce a greater safeguard against future pollution, although they are comparatively weaker disinfectants compared to free chlorine,
Doses of ammonia and chlorine used will depend upon the local characteristics of water.
Ammonia should be applying chlorine. properly applied and mixed with water about 20 min to 1-2 hours earlier than
Advantages:
1. They do not cause bad taste and odor when left as residual as is caused by chlorine alone.
2. They are very useful when phenols are present in water. The reaction of phenol with chloramines do not result in bad taste of water
3. Can remain in the water as residual for sufficient time